 |



  



2016 News Releases

Download

 | |  | | 

Puma Biotechnology Reports First Quarter 2016 Financial Results
LOS ANGELES, Calif., May 10, 2016 - Puma Biotechnology, Inc. (NYSE: PBYI), a biopharmaceutical company, announced financial results for the first quarter ended March 31, 2016.
Unless otherwise stated, all comparisons are for the first quarter 2016 compared to the first quarter 2015.
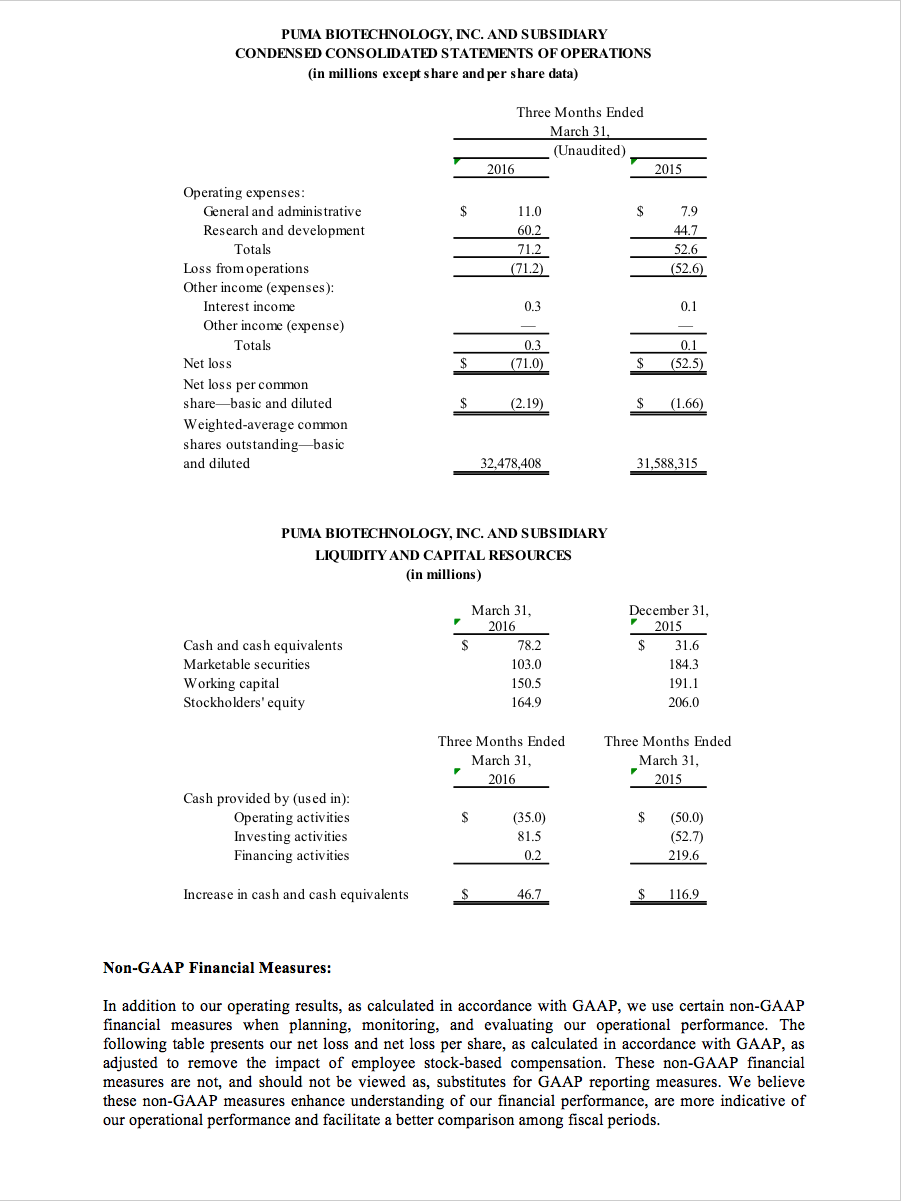
Based on accounting principles generally accepted in the United States (GAAP), Puma reported a net loss applicable to common stock of $71.0 million, or $2.19 per share, for the first quarter of 2016, compared to a net loss applicable to common stock of $52.5 million, or $1.66 per share, for the first quarter of 2015.
Non-GAAP adjusted net loss was $41.5 million, or $1.28 per share, for the first quarter of 2016, compared to non-GAAP adjusted net loss of $32.4 million, or $1.02 per share, for the first quarter of 2015. Non-GAAP adjusted net loss excludes stock-based compensation expense, which represents a significant portion of overall expense and has no impact on the cash position of the Company. For a reconciliation of GAAP net loss to non-GAAP adjusted net loss and GAAP net loss per share to non-GAAP adjusted net loss per share, please see the financial tables at the end of this news release. The Company anticipates that non-GAAP net loss will decrease in subsequent quarters due to an expected reduction in clinical trial expenses and the completion of the regulatory filings for neratinib for the extended adjuvant treatment of HER2-positive early stage breast cancer in the United States and Europe.
Net cash used in operating activities for the first quarter of 2016 was $35.0 million. At March 31, 2016, Puma had cash and cash equivalents of $78.2 million and marketable securities of $103.0 million, compared to cash and cash equivalents of $31.6 million and marketable securities of $184.3 million at December 31, 2015. The Company anticipates that net cash used in operating activities will be lower in subsequent quarters due to a reduction in the expenses described above.
“To date in 2016, we have achieved a number of key milestones, including publication of the results of the Phase III ExteNET trial of neratinib in the extended adjuvant treatment of HER2-positive early stage breast cancer in The Lancet Oncology, the publication of neratinib in the front-line treatment of HER2-positive metastatic breast cancer (NEfERT-T Phase II trial) in JAMA Oncology in April, and numerous presentations on neratinib in HER2 non-amplified breast cancer that has a HER2 mutation at the American Association for Cancer Research (AACR) Annual Meeting in April,” said Alan H. Auerbach, Chairman, Chief Executive Officer and President of Puma. “Our near term focus is on the filing of our regulatory submissions with the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for neratinib for the extended adjuvant treatment of HER2-positive early stage breast cancer, which we anticipate will occur in mid-2016 and the second quarter of 2016, respectively.
“We look forward to continuing our development of neratinib during 2016. We anticipate (i) submitting a New Drug Application (NDA) to the FDA in mid-2016 and submitting a Marketing Authorization Application (MAA) to the EMA during the second quarter of 2016 for neratinib for the extended adjuvant treatment of HER2-positive early stage breast cancer based on the positive ExteNET Phase III trial; (ii) reporting additional data from the Phase II trial of neratinib as an extended adjuvant treatment in HER2-positive early stage breast cancer using loperamide prophylaxis during the second quarter of 2016; (iii) reporting additional Phase II data from the FB-7 neoadjuvant HER2-positive breast cancer trial in the subgroup of patients who are MammaPrint High during the second quarter of 2016; (iv) reporting Phase II data from an investigator sponsored trial of neratinib in patients with HER2-negative breast cancer who have a HER2 mutation at the American Society of Clinical Oncology Annual Meeting in June 2016; (v) reporting data from the Phase III trial of neratinib in third-line HER2-positive metastatic breast cancer patients in either the fourth quarter of 2016 or the first quarter of 2017; (vi) reporting data from the Phase II trial of neratinib in metastatic breast cancer patients with brain metastases during the fourth quarter of 2016; and (vii) reporting data from the Phase II trial of neratinib plus fulvestrant in patients with HER2 non-amplified breast cancer that has a HER2 mutation during the fourth quarter of 2016.”
Operating Expenses
Operating expenses were $71.2 million for the first quarter of 2016, compared to $52.6 million for the first quarter of 2015.
General and Administrative Expenses:
General and administrative expenses were $11.0 million for the first quarter of 2016, compared to $7.9 million for the first quarter of 2015. The approximately $3.1 million increase resulted primarily from increases of approximately $1.2 million for stock-based compensation, $1.3 million for professional fees, $0.4 million for payroll and related costs, and $0.2 million for facility and equipment costs. These increases reflect overall corporate growth.
Research and Development Expenses:
Research and development (R&D) expenses were $60.2 million for the first quarter of 2016, compared to $44.7 million for the first quarter of 2015. The approximately $15.5 million increase resulted primarily from increases of approximately $8.2 million for stock-based compensation, $4.3 million for clinical trial expenses, $2.6 million for internal R&D and related expenses, and $0.4 million for consultants and contractors. We expect R&D expenses to decrease in subsequent quarters as we complete clinical trials and file for regulatory approvals for neratinib for the extended adjuvant treatment of HER2-positive early stage breast cancer in the United States and European Union.
About Puma Biotechnology
Puma Biotechnology, Inc. is a biopharmaceutical company with a focus on the development and commercialization of innovative products to enhance cancer care. The Company in-licenses the global development and commercialization rights to three drug candidates—PB272 (neratinib (oral)), PB272 (neratinib (intravenous)) and PB357. Neratinib is a potent irreversible tyrosine kinase inhibitor that blocks signal transduction through the epidermal growth factor receptors, HER1, HER2 and HER4. Currently, the Company is primarily focused on the development of the oral version of neratinib, and its most advanced drug candidates are directed at the treatment of HER2-positive breast cancer. The Company believes that neratinib has clinical application in the treatment of several other cancers as well, including non-small cell lung cancer and other tumor types that over-express or have a mutation in HER2.
Further information about Puma Biotechnology can be found at www.pumabiotechnology.com.
Forward-Looking Statements: This press release contains forward-looking statements, including statements regarding anticipated timing for regulatory filings and for the commencement and completion of various clinical trials and the announcement of data relative to these trials and the expected decrease in non-GAAP net loss, certain expenses and net cash used in operating activities in subsequent quarters. All forward-looking statements included in this press release involve risks and uncertainties that could cause the Company's actual results to differ materially from the anticipated results and expectations expressed in these forward-looking statements. These statements are based on current expectations, forecasts and assumptions, and actual outcomes and results could differ materially from these statements due to a number of factors, which include, but are not limited to, the fact that the Company has no product revenue and no products approved for marketing, the Company's dependence on PB272, which is still under development and may never receive regulatory approval, the challenges associated with conducting and enrolling clinical trials, the risk that the results of clinical trials may not support the Company's drug candidate claims, even if approved, the risk that physicians and patients may not accept or use the Company's products, the Company's reliance on third parties to conduct its clinical trials and to formulate and manufacture its drug candidates, the Company's dependence on licensed intellectual property, and the other risk factors disclosed in the periodic and current reports filed by the Company with the Securities and Exchange Commission from time to time, including the Company's Annual Report on Form 10-K for the year ended December 31, 2015. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The Company assumes no obligation to update these forward-looking statements, except as required by law.
Contact:
Alan H. Auerbach or Mariann Ohanesian, Puma Biotechnology, Inc., +1 424 248 6500
info@pumabiotechnology.com
ir@pumabiotechnology.com
Robert Flamm or David Schull, Russo Partners, +1 212 845 4235>
robert.flamm@russopartnersllc.com>
david.schull@russopartnersllc.com
# # # # #
(Financial Tables Follow)
Back to 2016 News Releases

|


Puma Biotechnology, Inc.
10880 Wilshire Blvd., Suite 2150
Los Angeles, CA 90024
424-248-6500 Main
424-248-6501 Fax

|



