
 |
|
|
|
2017 News Releases
The waterfall plot for the trial, % Change in Size of Target Lesions,” is shown in Figure 1, which is attached to this news release. In addition, the slides from the presentation are available on the Puma Biotechnology website. The safety results of the study showed that the most frequently observed grade 3 adverse events were diarrhea, nausea, thrombocytopenia and hypertension. More specifically, for the 21 patients with available safety assessments, grade 3 diarrhea was reported in 4 patients (19%), grade 3 nausea was reported in 3 patients (14%), grade 3 thrombocytopenia was reported in 3 patients (14%), and grade 3 hypertension was reported in 2 patients (10%). There was 1 dose limiting toxicity (DLT) at the 120 mg dose (1 of 6 patients), 3 DLTs at the 200 mg dose (3 of 8) and 2 DLTs at the 240 mg dose (2 of 3). Additional patients are currently being accrued at the 160 mg dose in order to define the recommended Phase II dose. Dr. Jame Abraham, Director of the Breast Oncology Program at Taussig Cancer Institute, Professor of Medicine at Cleveland Clinic, and principal investigator of the study, said, “We are encouraged by these initial findings and we will continue to enroll patients at the 160 mg dose to further evaluate the impact in this patient population.” Alan H. Auerbach, Chief Executive Officer and President of Puma Biotechnology, said, “We are pleased to see the high response rate of the combination of T-DM1 and neratinib in this patient population who were previously treated with both pertuzumab and trastuzumab in this study. We look forward to completing enrollment in the current cohort and moving this combination into Phase II trials.” About Puma Biotechnology Puma Biotechnology, Inc. is a biopharmaceutical company with a focus on the development and commercialization of innovative products to enhance cancer care. The Company in-licenses the global development and commercialization rights to three drug candidates—PB272 (neratinib (oral)), PB272 (neratinib (intravenous)) and PB357. Neratinib is a potent irreversible tyrosine kinase inhibitor that blocks signal transduction through the epidermal growth factor receptors, HER1, HER2 and HER4. Currently, the Company is primarily focused on the development of the oral version of neratinib, and its most advanced drug candidates are directed at the treatment of HER2-positive breast cancer. The Company believes that neratinib has clinical application in the treatment of several other cancers as well, including non-small cell lung cancer and other tumor types that over-express or have a mutation in HER2. Further information about Puma Biotechnology can be found at www.pumabiotechnology.com. Forward-Looking Statements: This press release contains forward-looking statements, including statements regarding the development of the Company’s drug candidates. All forward-looking statements included in this press release involve risks and uncertainties that could cause the Company's actual results to differ materially from the anticipated results and expectations expressed in these forward-looking statements. These statements are based on current expectations, forecasts and assumptions, and actual outcomes and results could differ materially from these statements due to a number of factors, which include, but are not limited to, the fact that the Company has no product revenue and no products approved for marketing; the Company's dependence on PB272, which is still under development and may never receive regulatory approval; the challenges associated with conducting and enrolling clinical trials; the risk that the results of clinical trials may not support the Company's drug candidate claims; even if approved, the risk that physicians and patients may not accept or use the Company's products; the Company's reliance on third parties to conduct its clinical trials and to formulate and manufacture its drug candidates; the Company's dependence on licensed intellectual property; and the other risk factors disclosed in the periodic and current reports filed by the Company with the Securities and Exchange Commission from time to time, including the Company's Annual Report on Form 10-K for the year ended December 31, 2016. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The Company assumes no obligation to update these forward-looking statements, except as required by law. Contact: Alan H. Auerbach or Mariann Ohanesian, Puma Biotechnology, Inc., +1 424-248-6500 info@pumabiotechnology.com ir@pumabiotechnology.com David Schull or Darren Chia, Russo Partners, +1-212-845-4271 david.schull@russopartnersllc.com darren.chia@russopartnersllc.com # # # # # 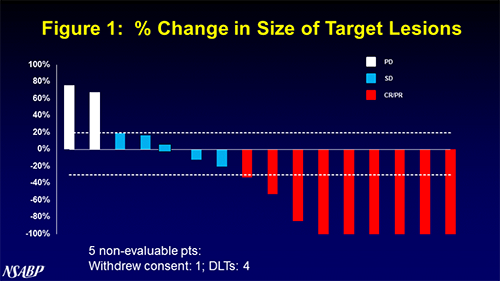
Back to 2017 News Releases |
 Puma Biotechnology, Inc. |

|
© 2024 Puma Biotechnology, Inc. | Privacy Policy | WA Privacy Policy | Terms of Service | Data Rights Request | Legal Notice | Site Map |